Venlo, Netherlands [press release]
QIAGEN's HPV testing technologies are a successful commercialization of the Nobel Prize in Medicine winner's research
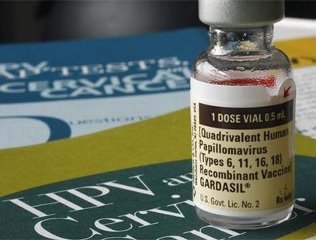
The Nobel Prize-winning discovery of the causal link between human papillomavirus (HPV) and cervical cancer has spurred lifesaving prevention technologies -- such as HPV testing and HPV vaccines -- that are helping to eradicate cervical cancer globally. The Nobel Prize Committee today announced that Professor Harald zur Hausen at the German Cancer Research Center in Heidelberg will receive this year's Nobel Prize in Medicine/Physiology for the discovery of certain human papillomaviruses (HPV) as the primary cause of cervical cancer. Expanding on Prof. zur Hausen's research, QIAGEN (Nasdaq: QGEN; Frankfurt, Prime Standard: QIA) developed and markets the digene(R: 49.52, +3.52, +7.65%) HPV Test, a screening test to detect the high-risk types of the HPV virus that cause cervical cancer. QIAGEN is also developing careHPV, a new HPV test specifically for use in regions of the world with scarce resources.
"Prof. zur Hausen has revolutionized cancer research and in particular cervical cancer research. His pioneering discoveries made the development of vaccines and novel diagnostics -- including our HPV testing platform -- possible, and today women around the world are benefiting from dramatically improved cervical cancer testing, treatment and prevention technologies. These innovations stem from the initial discoveries of Prof. zur Hausen. QIAGEN wholeheartedly congratulates Prof. zur Hausen on the Nobel Prize Award for his ground-breaking discovery. His vision and scientific excellence created a new paradigm for cancer research and led to such great strides in cervical cancer prevention," said QIAGEN CEO Peer Schatz.
The Nobel Prize winner's discovery that the human papillomavirus causes cervical cancer ushered in a new field of cancer-focused HPV genetics research. The identification of the causal role of the HPV virus in cervical cancer spurred research teams to identify the high-risk types of HPV most commonly linked to cervical cancer, then create molecular diagnostic tests to identify the presence of these high-risk types in cervical cells. Today, QIAGEN's digene HPV test is becoming a standard of care for cervical cancer prevention for use together with a Pap test in women age 30 and older, and the cervical cancer vaccine is helping protect populations of young girls from ever contracting two of the most prevalent strains of high-risk HPV.
"Prof. zur Hausen's seminal work spurred a new field of cancer research and, ultimately, a new generation of diagnostics and prevention technologies," said Dr. Attila Lorincz, Professor of Molecular Epidemiology at the Wolfson Institute of Preventive Medicine (UK) and Chairman of QIAGEN's Women's Health Scientific Advisory Board. "With the combination of Pap and HPV testing for women age 30+ and the availability of a vaccine to immunize young girls against certain strains of the virus before HPV exposure, the promise of the global eradication of cervical cancer is near. The development of these prevention technologies is the outgrowth of Prof. zur Hausen's discoveries. QIAGEN built on these discoveries and developed lifesaving testing technologies that today enable the identification of HPV -- the cause of cervical cancer -- before it can develop into disease, cause suffering and take lives." Dr. Lorincz led QIAGEN's development of the first ever diagnostic test for the high-risk types of HPV associated with cervical cancer.
Cervical cancer is the second-most-common cancer among women worldwide, and one of the most preventable ones. According to estimates 500,000 women are affected by cervical cancer every year causing around 250,000 deaths. The cancer is caused by sexually transmitted "high-risk" types of the human papillomavirus (HPV). Around 80 percent of women get an HPV infection at some point in their lives. However, in most cases, the infection goes away or is suppressed by the body without causing problems. It is only infections that persist that can cause abnormal cells to form that may develop into cervical cancer if not detected and treated early.
QIAGEN aims to make the benefits of HPV testing and early cervical cancer detection available to women across the globe. The digene HPV test and the careHPV test, currently in development, are screening tools that identify women most likely to have or develop cervical cancer by detecting the presence of the high-risk types of HPV which cause the disease. The digene HPV Test is the only FDA-approved and CE-marked test for the human papillomavirus, for use together with the Pap test in women 30 and older.
Labels: Cervical Cancer Research, HPV, HPV Vaccine