Recent Pap test recommendations and new vaccine approvals have brought cervical cancer screening into the spotlight and may have some women confused about what to do to protect themselves. Experts agree that routine screening is essential in the fight against cervical cancer, which is entirely preventable because it has a known cause-"high-risk" types of the human papillomavirus, or HPV.
According to the American Cancer Society, more than 11,000 women in the U.S. are diagnosed with cervical cancer each year. Eight out of 10 women are estimated to get HPV at some point in their lives, but the virus usually goes away on its own. However, infection with certain high-risk types of HPV may persist in some women and cause abnormal cells to develop into cervical cancer.
"We now have a full range of tools to protect future-and current-generations of women from developing cervical cancer, including vaccines, the Pap test and the HPV test," says Dr. Marie Savard, ABC News Medical Contributor and author of "Ask Dr. Marie: Straight Talk And Reassuring Answers To Your Most Private Questions." "Each tool has its own distinct function, so it's important to know the age recommendations, differences and how the tools can be used together to prevent cervical cancer."
Advances In Cervical Cancer Prevention
Screening technologies such as Pap and HPV testing allow for early detection of cervical cancer, while vaccines for girls and young women ages 9-26 can prevent future infections with certain cancer-causing HPV strains. Recently, the American Congress of Obstetricians and Gynecologists changed its Pap test screening recommendations to begin at age 21 and continue every other year. These new guidelines also say that women 30 and over can have a Pap test every three years when the Pap is "normal."
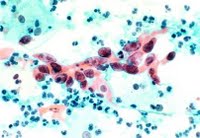
Doctors use Pap and HPV tests to determine a woman's risk for cervical cancer. A Pap looks for abnormal or precancerous cells caused by HPV, while the HPV test detects high-risk strains of the virus itself. HPV testing, performed together with the Pap in women age 30 and older, identifies women with high-risk HPV infections that can cause cervical cancer, enabling diagnosis and treatment to be put in place before cervical disease develops.
For women age 30 and older, who are at the greatest risk for cervical cancer, both tests can be performed at the same time and are covered by most insurance plans. Women with abnormal Pap results or who test positive for high-risk HPV should be monitored more closely by health care professionals so that any cell changes can be tracked and treated-before they can become cervical cancer.
"I had barely heard of HPV and yet here I was, diagnosed with cervical cancer at age 34," says Lori Stone, a cancer survivor and founder of the Pacific Northwest Cervical Health Coalition. "After years of normal Paps, an HPV test called the digene HPV Test alerted my doctor to do more follow-up, and we were able to catch my cancer at a stage when it was still very treatable."
Early detection of abnormal cervical cells is key so you can be closely monitored and treated accordingly. Take an active role in cervical cancer prevention! Be your own best advocate-ask your doctor what prevention tools are right for you: Pap testing, HPV testing and HPV vaccination.
Misread Pap Smear Test Failure to Diagnose Cervical Cancer Malpractice Claims Lawsuit AttorneysLabels: Cervical Cancer Awareness, Cervical Cancer Prevention, Cervical Cancer Risks