April 07, 2009 05:57 PM ET |
Deborah KotzIn response to my blog post yesterday on Farrah Fawcett and anal cancer, I received an interesting E-mail from a reader who had been diagnosed with cervical cancer 10 years ago as a result of an infection with human papillomavirus. She had heard of the study published in last week's New England Journal of Medicine showing that a newer DNA test for HPV saves more lives from cervical cancer than traditional Pap smears. Here's an excerpt from her letter:
"I was hoping to get your take on the new HPV DNA test that is being touted as the replacement to the Pap smear. Reason I ask is that currently, HPV testing is ONLY recommend for women over 30 and the fact that they would do away with the Pap in favor of HPV DNA testing has many of us in the survivor community wondering what this all means. Will women under 30 get the HPV test? Will Paps be gone and HPV DNA testing only exist for women over 30? What does it all mean?"
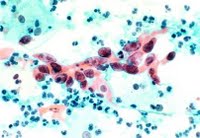
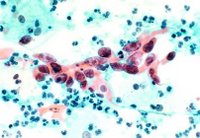
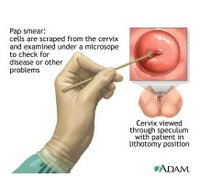
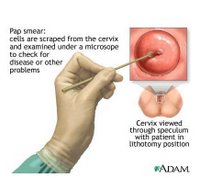
The American College of Obstetricians and Gynecologists currently recommends annual Pap screens—which involve taking a small scraping of cells from the cervix—for young women about three years after they begin having sex or by age 21, whichever comes first. Starting at age 30, women have three options: If they have had three negative Pap results, they can continue annual Pap testing or switch to screening every two or three years. They can also add screening with an HPV-DNA test, which is not a blood test but also involves taking a small sample of cells from the cervix. (Those with HIV or certain other conditions should continue annual screening.)
I asked Kenneth Noller, past president of ACOG and chair of the department of obstetrics and gynecology at Tufts Medical Center, if he could tell me what this latest research means for cervical cancer screening.
Will this new study change anything? Will Pap smears indeed become a thing of the past?
"One study never does anything by itself, but there are a lot of studies showing HPV tests are better than doing cytology on a Pap smear," says Noller. "But that's if you're comparing a one-time Pap smear with a one-time HPV test, which was how the latest study was performed." The two tests perform about equally, on the other hand, when a woman receives one or the other repeatedly over several years. That being said, Noller predicts that Pap smears will become less common in women over 30 as more doctors turn to HPV testing.
What about women under 30? Shouldn't they have HPV tests too?
Not unless they have an abnormal Pap smear, Noller says. Widespread HPV testing in that younger group, he says, could lead to "unnecessary testing, treatment, and worry." While about 15 to 20 percent of women under 30 test positive for a high-risk HPV strain, about 90 percent of those infections vanish without treatment. For this reason, doctors tend to perform HPV tests in younger women only when there's an abnormal Pap result and they need to determine whether further procedures are warranted. Women over 30, on the other hand, are less likely to be infected with HPV, but those who are infected are more likely to have a persistent infection. If HPV tests completely replace Paps in women over 30, Noller says he's not sure how many labs will remain in business to keep analyzing cells from Pap tests.
So, what should women do?
Women under 30 should stick with Pap smears, and those over 30 should speak to their doctor about HPV testing. The HPV test could be particularly useful if it's been several years since a woman's last screening. For now, there's no reason to panic if you're a younger women. Paps are still widely available.
Labels: Cervical Cancer Screening